CANTHARIDIN: A Historical Overview and Synthetic Approach
April 11, 1994
By: Paulus W. Wanandi
Class of 1995
TABLE OF CONTENTS
| I. | Introduction | 1 |
| II. | Origin, Toxicity, and Uses | 2 |
| III. | Historical Background | 4 |
| IV. | Synthesis | 7 |
| V. | Further Work | 13 |
| VI. | Conclusion | 18 |
| VII. | References | 19 |
| VIII. | Appendix | 21 |
I. Introduction
Cantharidin (I) is the active ingredient of cantharides, a toxic preparation isolated from the dried bodies of blister beetles (Lytta vesicatoria or Cantharis vesicatoria), which, besides its notoriety as the reputed aphrodisiac in "Spanish fly," has found commercial applications as a potent vesicant (blister-causing agent), counterirritant, and in the removal of benign epithelial growths such as common warts.
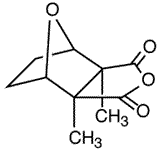
I
Since its initial isolation in crystalline form by the French pharmacist Robiquet in 1810,1 cantharidin has been subject to extensive structural and synthetic investigations, owing to its relatively simple structure and extraordinary physiological properties. The seemingly-obvious synthesis of this molecule via a concerted [4+2] Diels-Alder cycloaddition reaction between furan and dimethylmaleic anhydride, followed by hydrogenation, was investigated as early as the 1920s,2,3 but such a direct synthetic approach failed. Although some early attempts at the stereospecific syntheses of 1 had been successful,4-6 the length and complexity of these efforts stand in sharp contrast to the uncomplicated structure of I. It is only recently that a short and efficient total synthesis of I was achieved by a Diels-Alder reaction, carried out under high pressure between furan and a dihydrothrophene anhydride, a cyclic sulfide derivative of dimethylmaleic anhydride.7
This report will examine the historical background of cantharidin and its recent synthesis. The biological origin (including biosynthesis), toxicity, and practical uses of the compound will be briefly mentioned. Then, the historical background of the compound, particularly the early investigations that led to the determination of the correct structure and the early attempts--successful or otherwise--at its synthesis, will be described. Finally, the most recent synthesis of cantharidin will be described in detail, including the problems associated with it, its preparative scale, the advantages it has over previous syntheses, and the current efforts being made for its improvement. A new synthesis of the compound will also be suggested.
II. Origin, Toxcity, and Uses
Cantharidin (1) is naturally found in various species of blister beetles (family Meloidae), the most familiar of which is the "spanish fly" Lytta vesicatoria (or Cantharis vesicatoria). Used as a defensive substance in these beetles, cantharidin is biosynthesized in the adult male beetles during mating and is completely transferred into the females (which do not produce cantharidin on their own) through the sex organs.14 It was also found that the male beetles continue to produce cantharidin after mating, an evidence which suggests that the biosynthesis of cantharidin in the male beetles is stimulated during copulation and occurs in the accessory glands of the male sexual organs.14 Labelling studies using radioactive isotopes of carbon (14C) and hydrogen (3H), and nonradioactive oxygen (18O), with mass spectrometry have indicated that the biosynthesis proceeds by an unprecedented degradation of farnesol (II),15
Commercially, cantharidin is available as CANTHARONE®, a cantharidin (0.7%) collodion used for the removal of benign epithelial growths such as warts and molluseum contagiosum.19 The apparently original characteristics of cantharidin-induced inflammation (absence of involvement from immunological processes) may also make it useful for the testing of anti-inflammatory and anti-allergic drugs.20 The reputation of cantharidin as an aphrodisiac upon ingestion is widely accepted due to the resulting irritation of the urethra (male genital duct), which may result in priapism (persistent erection of the penis).18 However, as already mentioned above, its ingestion is dangerous, sometimes lethal.21
VII. References
| (1) | Robique. M. Ann. Chim. 1810, 76, 302-307. |
| (2) | von Bruchhausen, F.; Bersch, II. W. Arch. Pharm. Ber. Disch. Phurm. Ges. 1928, 266, 697-702. |
| (3) | Diels. O.; Alder, K. Ber. 1929, 62, 554-562. |
| (4) | Ziegler, L.; Schenck, G.; Krockow, E. W.; Siebert, A.; Wenz, A.; Weber, H. Justus Liebigs Ann. Chem. 1942. 551, 1-79. |
| (5) | Stork, G.; van Tamelen, E. E.; Friedman, L. I.; Burgstahler, A. W. J. Am. Chem. Soc. 1953, 75, 384-392. |
| (6) | Schenck, G.; Wirtz, R. Naturwissenshaften 1953, 40, 531. |
| (7) | Dauben, W. G.; Kessel, C. R.; Takemura, K. H. J. Am. Chem. Soc. 1980, 102, 6893-6894. |
| (8) | Dauben, W. G.; Krabbenhoft, II. O. J. Am. Chem. Soc. 1976, 98, 1992-1993. |
| (9) | Gladysz, J. A. CHEMTECH 1979, 372-377. |
| (10) | Jurezak, J.; Kozluk, T.; Filipek, S.; Eugster, C. H. Helv. Chim. Acta 1982, 65, 1021-1024. |
| (11) | McCormick, J. P.; Shimmyozu, T. J. Org. Chem. 1982, 47, 4011- 4012. |
| (12) | Matsumoto, K.; Hashimoto, S.; Ikemi, Y.; Otani, S.; Uchida, T. Heterocycles, 1986, 24, 1835-1839. |
| (13) | Grieco, P. A.; Nunes, J. J.; Gaul, M. D. J. Am. Chem. Soc. 1990, 112, 4595-4596. |
| (14) | Sierra, J. R.; Woggon, W. D.; Schmid, H. Experientia 1976, 32, 142-144. |
| (15) | McCormick, J. P.; Carrel, J. E.; Doom, J. P. J. Am. Chem. Soc. 1986, 108, 8071-8074. |
| (16) | Peter, M. G.; Woggon, W. -D.; Schmid, H. Helv Chim. Acta 1977, 60, 2756-2762. |
| (17) | Graziano, M. J.; Casida, J. E.; Waterhouse, A. L. Biochem. Biophys. Res. Comm. 1987, 149, 79-85. |
| (18) | Waddell, T. G.; Jones, H.; Keith, A. L. J. Chem. Educ. 1980, 57, 341-342. |
| (19) | Physicians' Desk Reference to Pharmaceutical Specialties and Biologicals; Medical Economics, Inc.: New Jersey, 1970; p 1727. |